Skin Substitute Protocol & Success Stories
Wound Care Solutions

CTP/CAMP Graft Success
Wound Bed Preparation Fundamentals
This comprehensive guide explores the essential principles of wound bed preparation and their direct impact on cellular and tissue-based product outcomes. Clinicians will learn systematic approaches to assess wound readiness, manage bioburden, control moisture, and optimize the healing environment. Through evidence-based protocols and practical techniques, this resource provides the critical knowledge needed to maximize graft adherence, vascularization, and successful integration of CTPs/CAMPs across various wound types.
Classification of Persistent Tissue Injuries
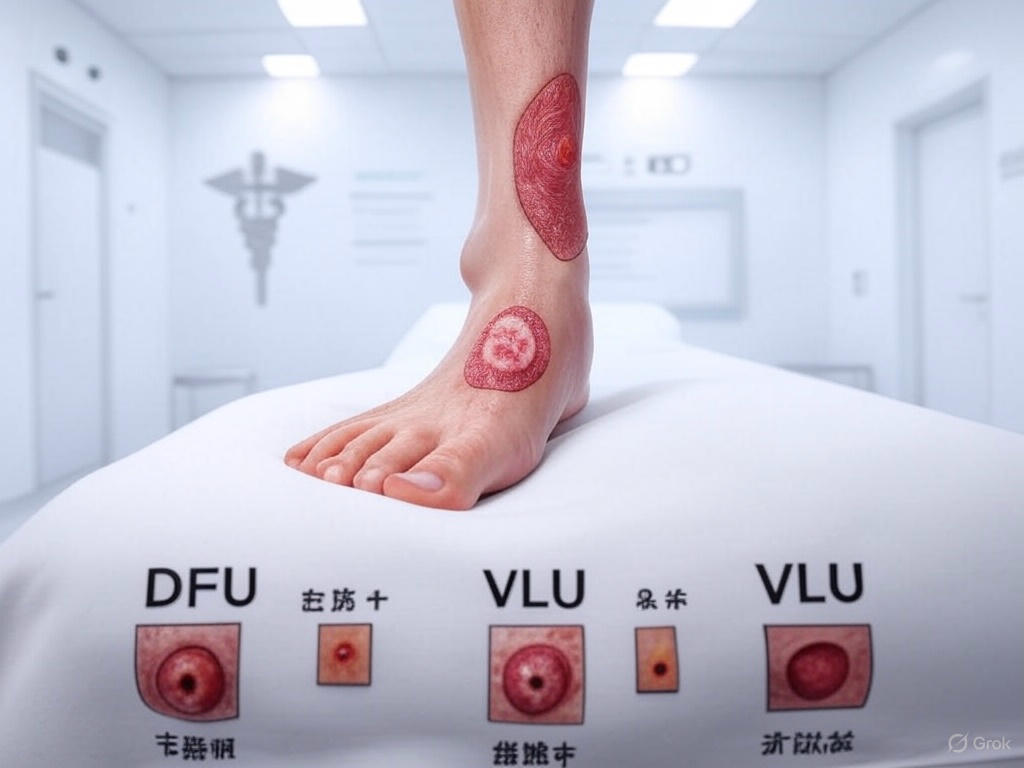
Types of Chronic Wounds
This overview identifies the primary categories of chronic wounds seen in clinical practice, each with distinct pathophysiology and treatment requirements. Understanding these classifications is essential for targeted assessment and effective management strategies.
- Venous Leg Ulcers (VLU)
- Arterial
- Diabetic Foot Ulcers (DFU)
- Pressure
- Trauma
Phases of Wound Healing
Acute Process
Body advances through healing phases in orderly and timely manner
Chronic Status
Wound does not progress as expected, becoming classified as chronic

Local Factors
Pressure, trauma, edema, excessive bacteria and decreased oxygen delay healing
Systemic Factors
Age, chronic disease and poor nutrition impede normal healing
Dedicated to Building Excellence.
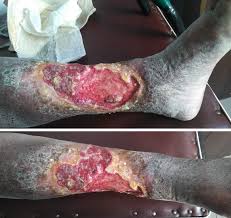
Chronic Wounds
- Primarily Diabetic Foot Ulcers (DFUs) & Venous Leg Ulcers (VLUs)
Other Wound Applications
- Chronic Pressure Ulcers
- Post-Surgical Wounds
- Disease-State Wounds
- And more...
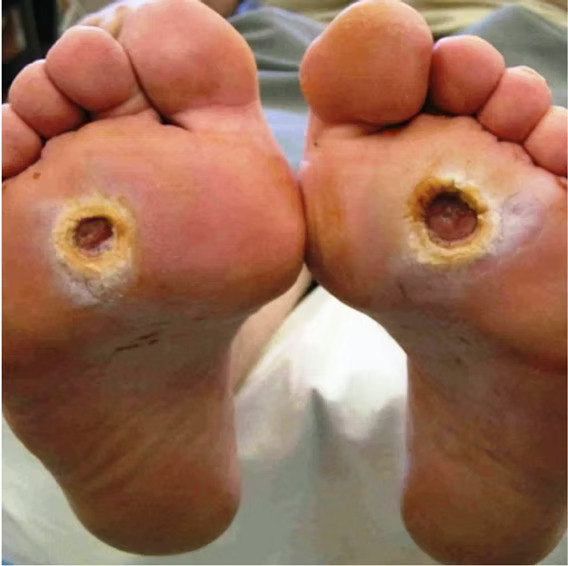
Diabetic Foot Ulcers (DFUs)
Venous Stasis, Chronic Scalp Ulcers (VLUs)
- Our Process
Phases of Wound Healing
Wound healing unfolds in distinct phases: clot formation, inflammation, proliferation, and remodeling. This timeline outlines key events, cellular and vascular responses, and the challenge of chronic wounds, which often stall in the inflammatory phase, aiding effective wound management strategies.
Injury: 0 days
Inflammatory
Phase: 3 days
Proliferation
Phase: 7 days
Remodeling Phase:
3 weeks to 1 year
Other Factors Affecting Wound Healing
- Certain factors may affect the quantity, timing, and activity of the cytokines, growth factors, and MMPs that play a role in wound healing
- MMP (Matrix Metalloproteinase): enzymes that break down proteins in the wound bed
- Certain growth factors, including PDGF, EGF, and TGFb, are found in decreased levels in chronic wounds compared to acute wounds
- In addition, bacteria found in chronic wounds
- In some cases, these substances may be present, but not available to the wound for healing
- Growth factors known as Cytokines are proteins that control the signaling properties of the wound bed.
- Growth factors are mediators that tell cells how to behave, affecting migration, reproduction, and cell death.
- Growth factors bind to ECM in order to be activated. Therefore, organized ECM plays a critical role in regeneration.
- Certain growth factors, including PDGF, EGF, and TGFb, are found in decreased levels in chronic wounds compared to acute wounds.
- In some cases, these substances may be present, but not available to the wound for healing.


T.I.M.E Management Approach to Graft Prep
T
- Manage Tissue Quality ensuring it is viable.
I
- Manage the bacterial balance to Infection.
M
- Control the Moisture and exudate.
E
- Manage the cooperation of the wound Edges.
Overview of Wound Bed Preparation Treatments Types

Categories of Grafts
- Autograft: Patient’s own tissue
- Allografts: Human substrate
- Xenograft: Animal substrate
- Synthetic: Laboratory derived
- Placental: Amnion, Chorion
Placental Membranes
- Amnion: Comprised of the innermost layer of the placenta; lines the amniotic cavity
- Chorion: Outer placental layers

Universal Patient Health Considerations of Address Before Graft Treatment

What is needed for LCD Compliance?
- Nutrition
- Blood albumin levels (‘building blocks’ for repair, colloid osmotic pressure -)
- Perfusion and oxygenation
- Immune status
- Pain(causes vasoconstriction)
- Corticosteroids(depress immune function)
- Smoking Cessation
- Control of Edema
- Blood glucose level(simpaired white cell function)
- Hydration status(dehydration slows metabolism)
- Wound not involving exposed: Tendon, Muscle, Joint Capsule, Bone or Sinus Tract
- Wound larger than 1sq cm in size
The Policies Focus on Two Main Conditions
Diabetic Foot Ulcer (DFU) and Venous Leg Ulcer (VLU) since that is the majority of use for skin subs. Therefore, this influences other applications:
- Chronic wounds always need to show treatments tried and failed at least 30 days SOC as per definition of “chronic”
- Adequate blood flow (typically ABI above 0.65 in the last 60 days, however any form documenting blood flow to the wound is acceptable)
- Controlled diabetes (gold standard is A1C levels under 12% in the last 90 days), however any adequate medical management of the condition is acceptable
- Presence of a venous stasis ulcer for at least 3 months
- Unresponsive to appropriate wound care for at least 30 days with documented compliance
- Adequate blood flow and diabetic, controlled diabetes
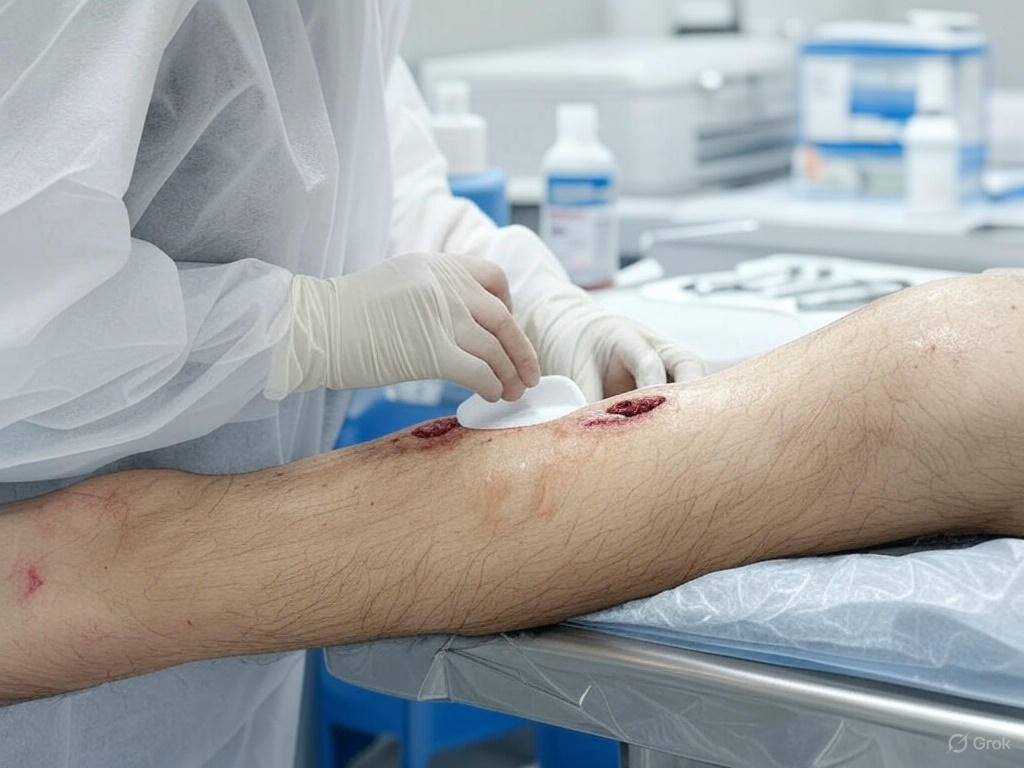
Additional Considerations for Skin Substitute Use
A description of the wound(s) must be documented at baseline (prior to beginning conservative treatment) relative to size, location, stage, duration, and presence of infection, in addition to the type of treatment given and the patient’s response.
- This information must be updated in the medical record throughout treatment.
- Measurements pre- and post-debridement of the wound bed should be documented.
- Wound description must also be documented pre- and post-treatment with the skin substitute graft being used.
- If obvious signs of worsening or lack of treatment response are noted, continuing treatment with the skin substitute would not be considered medically reasonable and necessary without documentation of a reasonable rationale for doing so.
The amount of utilized and wasted skin substitute must be clearly documented in the procedure note with the following minimum information:
- Date, time, and location of the ulcer treated
- Name of the skin substitute and how the product was supplied
- Amount of product unit used
- Amount of product unit discarded with the reason for wastage
- Manufacturer’s serial/lot/batch or other unit identification number of the graft material. If the manufacturer does not supply unit identification, the record must still document such. Consider including pictures of graft packaging.
